Back to the COVID-19 First Doses First Project Overview.
- Introduction
- How would one calculate the efficacy then?
- What does it mean?
- What should we do with this information?
- Post Scriptum
- Appendix
- What about other vaccines (Moderna, AZ, Sputnik,…)
- Sources
Introduction
People think that the Pfizer vaccine against COVID-19 only protects against the illness after the second shot has been administered. When looking at the data from the phase 3 trial, this is obviously wrong. In this post I will try to clarify some of these misunderstandings as easy as possible.
The following picture is the relevant plot from the publication1. The two curves (blue & red) show the COVID-19 incidence in the placebo (blue) and treatment (red) groups.
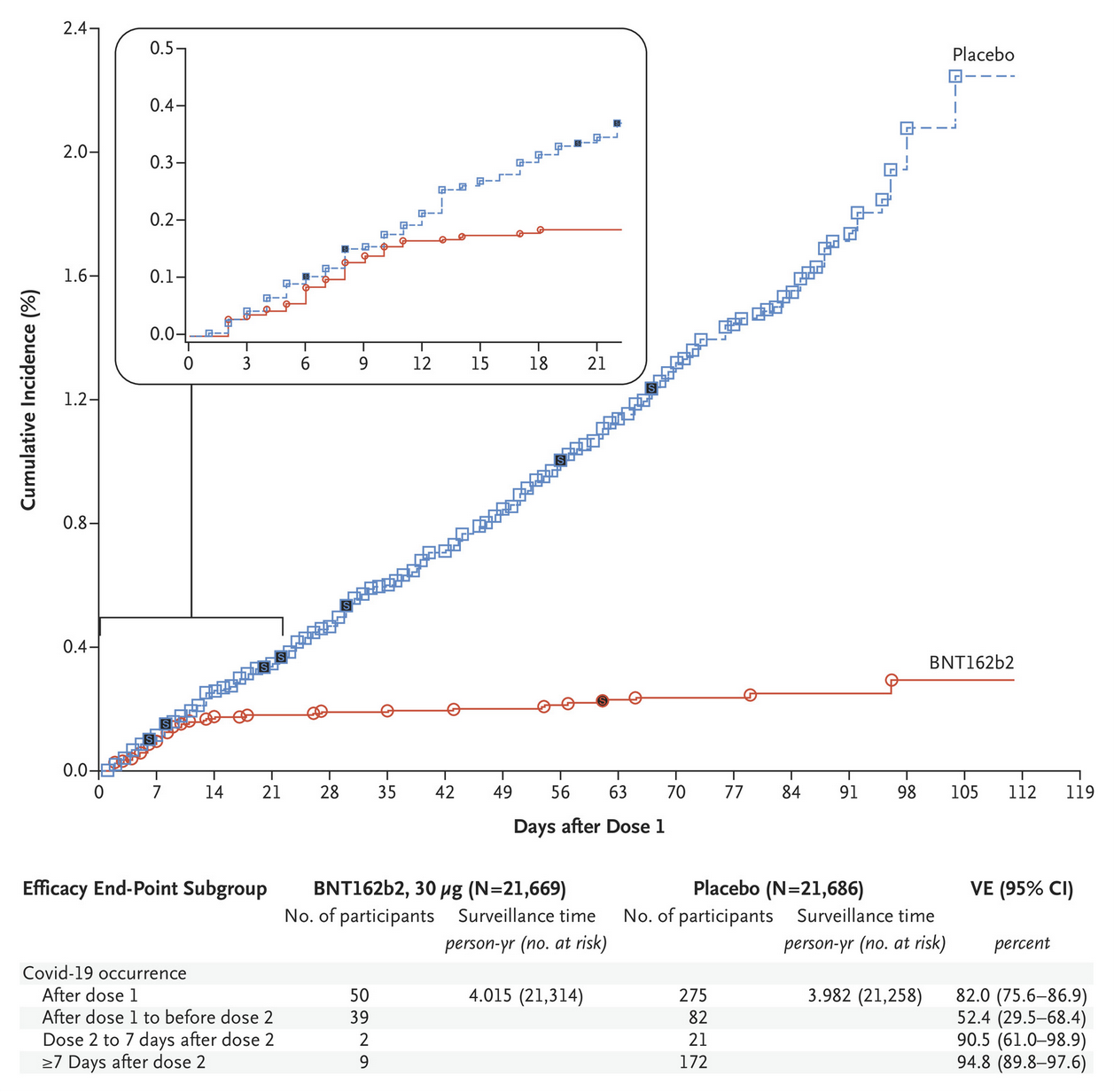
If the vaccine doesn’t work, one would expect both lines to have a similar slope. If it works, the slope of the treatment group will be less steep than that of the placebo group. Efficacy is calculated as the fraction of the gradients of the two slopes.
This means: If the placebo group rises by 10 units per day and the vaccine group rises by 2 units per day, this translates to a 80% efficacy (as a group that would have had 10 infections without vaccine only has 2 infections, a reduction of 80%).
The table below the plot shows the efficacy of various time-intervals:
- After dose 1 (day 0 to the end): 82%
- After dose 1 and before dose 2 (day 0 to 21): 52.4%
- Dose 2 to 7 days after dose 2 (day 21 to 28): 90.5%
- More than 7 days after dose 2 (day 28 to the end): 94.8%
One would think that second and third/fourth of these numbers correspond to the efficacy of 1 dose vs 2 doses but this is wrong because it neglects how vaccinations work as well as the incubation period.
The incubation period
If a person is exposed to the virus on day X, it takes a few days (4-5, up to a week) until this person gets sick. More importantly, even a PCR test wouldn’t register the virus directly after exposure. An antigen test would detect it a few days after exposure.
Therefore it’s safe to assume that people that tested positive in the first days after dose 1 were already infected before they were vaccinated.
How vaccinations work
A vaccine stimulates the immune system to produce protection against some virus. As this process takes some time when a person gets infected with e.g. the flu (approx. 1 week afaik) it’s safe to assume that it also takes some time when the immune system is triggered with a vaccine and not a real live virus. I don’t know how long it takes for the immune system to react to this specific vaccination, but it’s not necessary to guess, as the data from the trial actually shows it.
If you look at the red and blue curves right after the injection, they follow a very similar trajectory until day 10-12 (the red one is slightly below the blue one even earlier but this is likely random noise).
From then one, there is a gap between the curves that gets continuously larger.
How would one calculate the efficacy then?
The same arguments as above also apply to the second dose. People that receive the 2nd dose on day 21 and test positive on day 23 were likely already infected before receiving the 2nd dose. People that test positive on day 26 might have been infected after receiving the second dose but it’s likely that their immune system didn’t yet fully react to the second stimulation, meaning their level of protection against a severe case of COVID-19 (if it exists) is still from the first dose.
Therefore, it’s likely better to look at the slope between days 14 and 28 to get the efficacy of the first dose and at day 28 and later for the efficacy of both shots.
I used the professional medical data analysis tool MS Paint™ to remove the first ~10 days and put some slopes in there to estimate the efficacy of one dose (green line) and two doses (purple line).
The result can be seen here:

What does it mean?
- The green line is already way less steep than the blue one, i.e. the first dose is likely already enough to prevent symptomatic cases (afaik there wasn’t a severe case of COVID in the population vaccinated with 1 dose after the initial period where the vaccine isn’t effective yet during any of the vaccine trials)
- The purple line is slightly less steep than the green one, but compared to the blue one this increase is marginal. Data from the NHS says that the increase in immunity from the 2nd dose is approx 1/15th of the increase of the first dose (89% vs 95%)2
What should we do with this information?
Put pressure on politicians to stop wasting 50% of vaccine doses on people that are already >80% immune and use it for persons that are still 0% immune. Keith A Moyse from Oxford3 has calculated the optimal vaccination strategy taking these things into account, basically it is:
- Give people N to N+5 years old one dose (starting with old people)
- Give people in the brackets 15 years below the first dose
- Then give the old people the second dose
If anyone wants to point out obvious mistakes I am making or has anything else to say to me, feel free to contact me on twitter or wherever else you would prefer (contact information is available on the main page of this blog).
Post Scriptum
After I’ve written this post, I stumbled on a Twitter Thread by Stanford Associate Professor Michael Lin that lays out the same argument (though he did so in January 2021).
In addition to what I got from the data he said that the way the efficacy rates are calculated in the paper are not actually standard practice (which I assumed) but are a mistake in the trial design.
To clarify what this means:
- A pharmaceutical company makes a mistake in their trial design (which even an amateur with no prior knowledge can spot)
- They still release the paper as it is (likely because it’s not allowed to change after registering a protocol)
- Lots of highly credentialed people as well as a complete amateur (I) notice these issues from a cursory glance at the paper (read abstract, look at figures & tables).
- The FDA & EMA are not allowed to do anything about it, they just put their stamp of approval on the studies as they are
- The politicians defer their decisions to ““experts”” in charge of the vaccination program
- The “”””"”experts”””””” don’t notice these obvious things (I assume that they are either too lazy to actually look at the studies or they are not able to understand them or a combination of both) and just defer to the EMA.
Appendix
What about other vaccines (Moderna, AZ, Sputnik,…)
The same reasoning with slightly different numbers also applies to the Moderna and AZ vaccines. Their papers don’t have a plot that is as nice as the one from the Biontech paper, therefore I used that one for this post.
But if you’re interested, here’s the plot from Moderna 4 (Fig. 3b in the paper)

What are other things that should be considered?
Common arguments against a single dose vaccination strategy are immune escape and long time protection. I answer these arguments in a separate post.
Sources
-
comparison between 1 and 2 dose prioritisation for a fixed number of doses ↩
-
In order to save as many lives as possible, rollout of the coronavirus vaccine should prioritise age groups so that the importance of giving younger ages their first vaccination is balanced against the importance of giving older ages their second ↩